On March 16th, 2023, after thirty years of research started in France with the support of ELA, the first boy in the world received the FDA approved gene therapy for the cerebral form of adrenoleukodystrophy.
Following a positive newborn screening, 6-year-olds Conner Hess from New York was followed closely to monitor a possible evolution of the disease. Last September, an MRI scan revealed the dreaded signs of brain changes suggesting of progression toward cerebral X-linked adrenoleukodystrophy that causes neurological devastation. Preparing for a bone marrow transplant (or stem cell transplant), which provides healthy donor stem cells to produce the lacking protein, the search for a good matching donor proved unsuccessful.
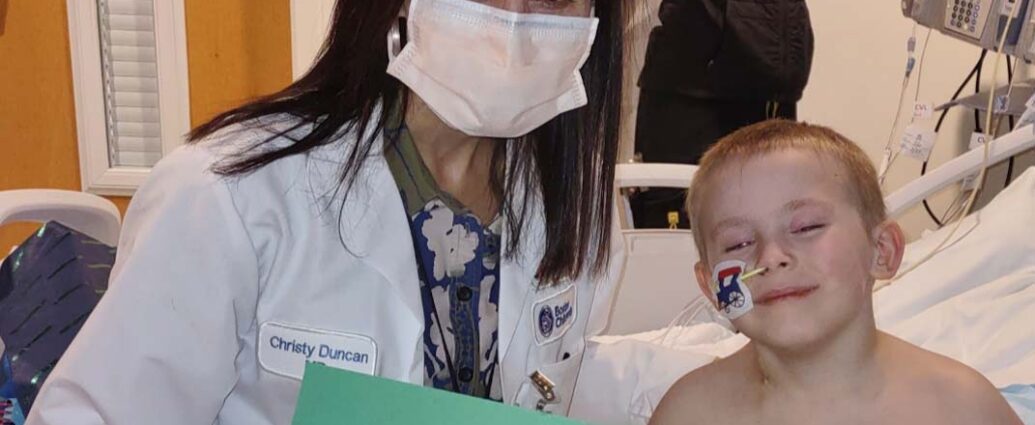
Conner’s neurologist in New York referred him to Boston Children’s, where Drs. Christine Duncan, clinical director of Boston Children’s Gene Therapy Program, and Florian Eichler, director of the Center for Rare Neurological Diseases at Massachusetts General Hospital, work together to evaluate and care for patients with cerebral adrenoleukodystrophy. Just weeks before, the U.S. Food and Drug Administration had approved SKYSONA (elivaldogene autotemcel), a gene therapy for cerebral adrenoleukodystrophy. In this approach, bone marrow is to be taken not from the donor but from the child himself, treated with a virus carrying the gene for the ALD protein, then reinfused.
In January 2023, Conner underwent a procedure to remove some of his stem cells, which were then processed in laboratory and treated with the healthy ALD gene. In March, he returned to the hospital to begin chemotherapy, needed to prepare the bone marrow for the transplanted stem cells. Conner Hess is the first kid in the world to receive the one-time treatment outside of a clinical trial. Seventeen years after the treatment of the first patient in France by the teams of Pr. Aubourg, the now costing $3 million the therapy will likely save his life.
The boy remains at the hospital for three to four weeks after the infusion and will be monitored closely for the next 15 years. But if results from the clinical trials that led to approval of the treatment are any indication, it will give him a chance to survive and thrive.